Research
Green Hydrogen
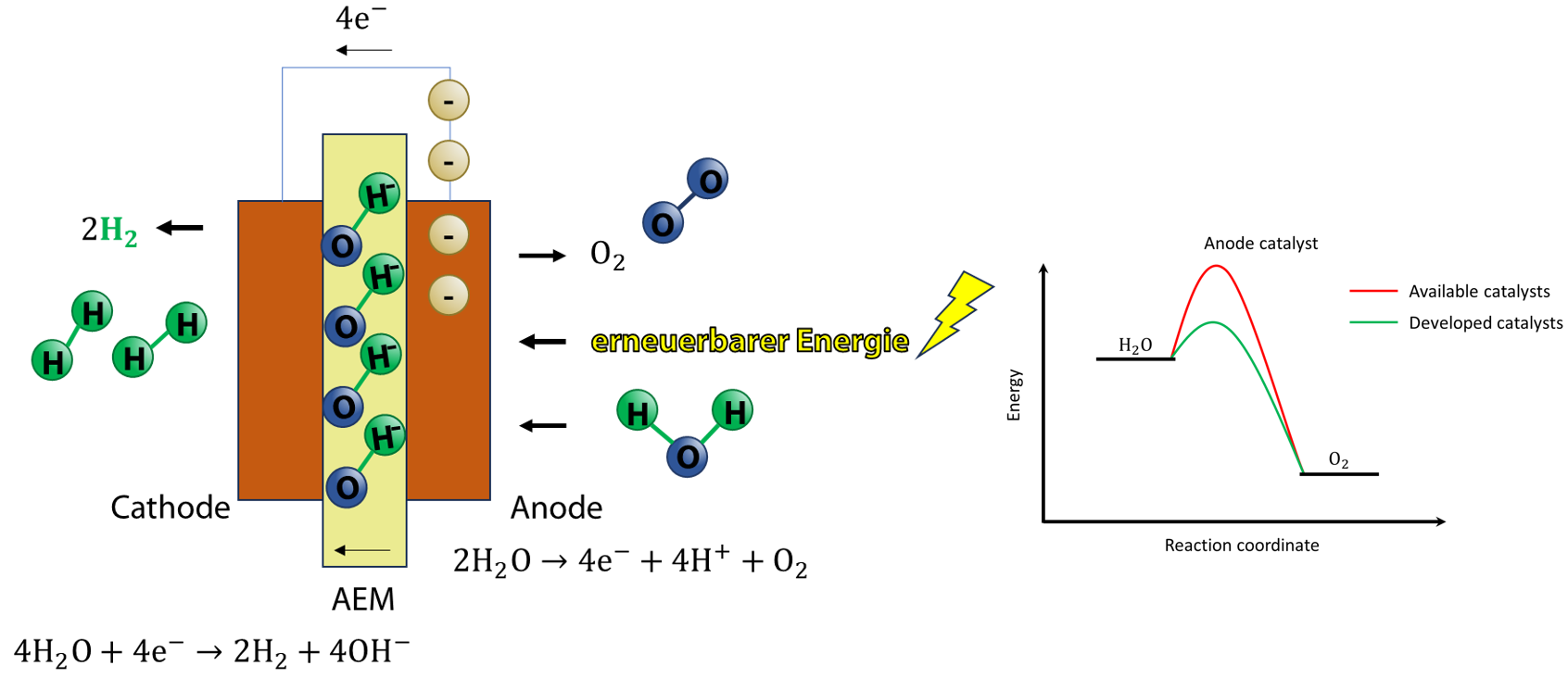
Green hydrogen (GH2) is hydrogen produced from water electrolysis using renewable energy sources, a process considered to be environmentally friendly as no greenhouse gases are emitted. In particular, anion exchange membrane electrolyzers (AEMEL) are an attractive electrolysis technology that combines the key advantages of both proton exchange membrane electrolyzers (PEMEL – e.g., high current density and fast start up time) and alkaline electrolysis (AEL – e.g., low cost and non-precious metal catalysts) . However, the sluggish kinetics of the oxygen evolution reaction (OER) in these systems lead to a high overpotential, which hampers its applicability at industrial scales.
The group of Electrochemical Process Engineering is partner in a collaboration between Germany and New Zealand that aims to develop highly efficient anode catalysts to reduce the cell voltage of the water electrolysis reactor, with particular emphasis on reducing the overpotential caused by the OER. The electrodes, which are based on nickel and manganese, are fabricated using the dynamic hydrogen bubble template method, which results in a porous electrode structure .
Lithium-Ion Batteries
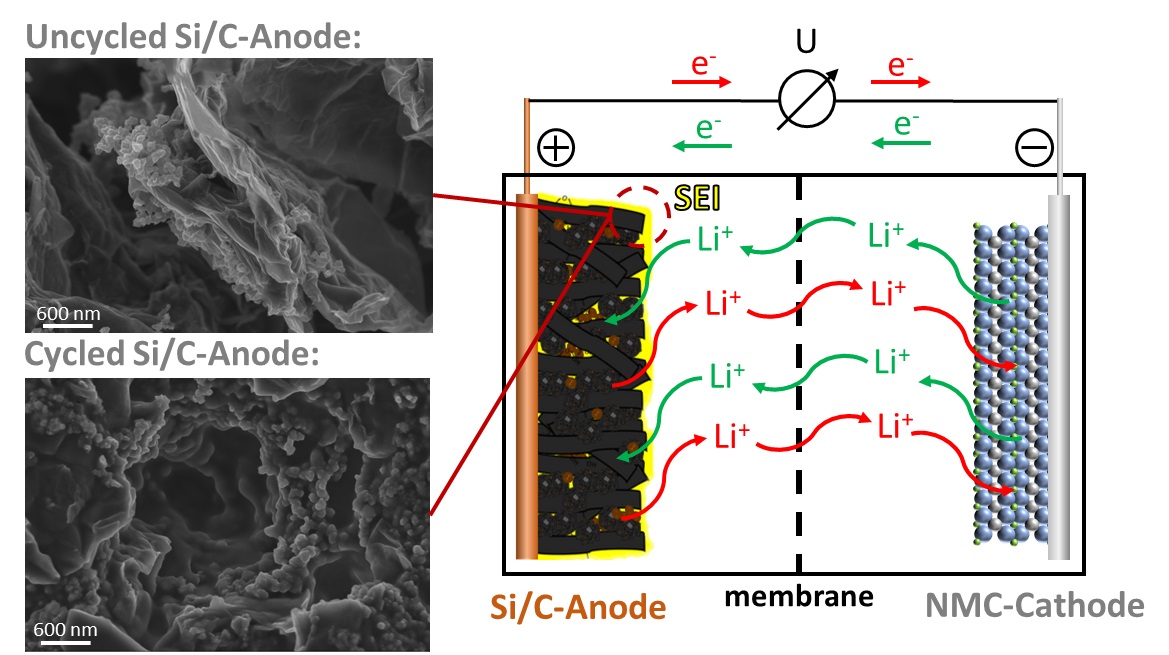
Due to their high volumetric and gravimetric energy density as well as cycle stability, lithium-ion batteries (LiBs) are used in mobile devices such as laptops or smartphones (among other things). In recent years, however, their use in larger applications such as motor vehicles has been increasing. Commercial LiBs usually consist of a carbon-based electrode on the anode side and a combination of different transition metals such as nickel or cobalt with lithium on the cathode side. The electrodes are separated by an ion-conductive but electrically insulating separator, and the space between the electrodes and membrane is usually filled with a liquid lithium-containing organic electrolyte to enable ion conduction, with some applications using a solid-state electrolyte instead.
As part of the Bavarian Center for Battery Technology (BayBatt), we are engaged in the successive further development of individual battery components. The focus of our research here is on the synthesis of silicon-carbon composites as novel anode materials, the electrochemical characterization of glass-based thin-film separators, and the development of in-situ and in-operando analysis strategies to investigate the complex electrochemical processes at the electrode-electrolyte interface in more detail.
Redox flox batteries
Redox flow batteries store electrical energy chemically in the form of electrolyte solutions stored in tanks. The main advantage over other storage systems is that the storage capacity can be influenced by the volume of the electrolyte alone, regardless of the cell size. In our projects we want to better understand the aging of porous carbon electrodes and produce improved C-C composite electrodes. In particular, we also use electroanalytical methods (RDE, ACCV, DRT-EIS) to investigate the relationship between structure and activity.
Fuel cells
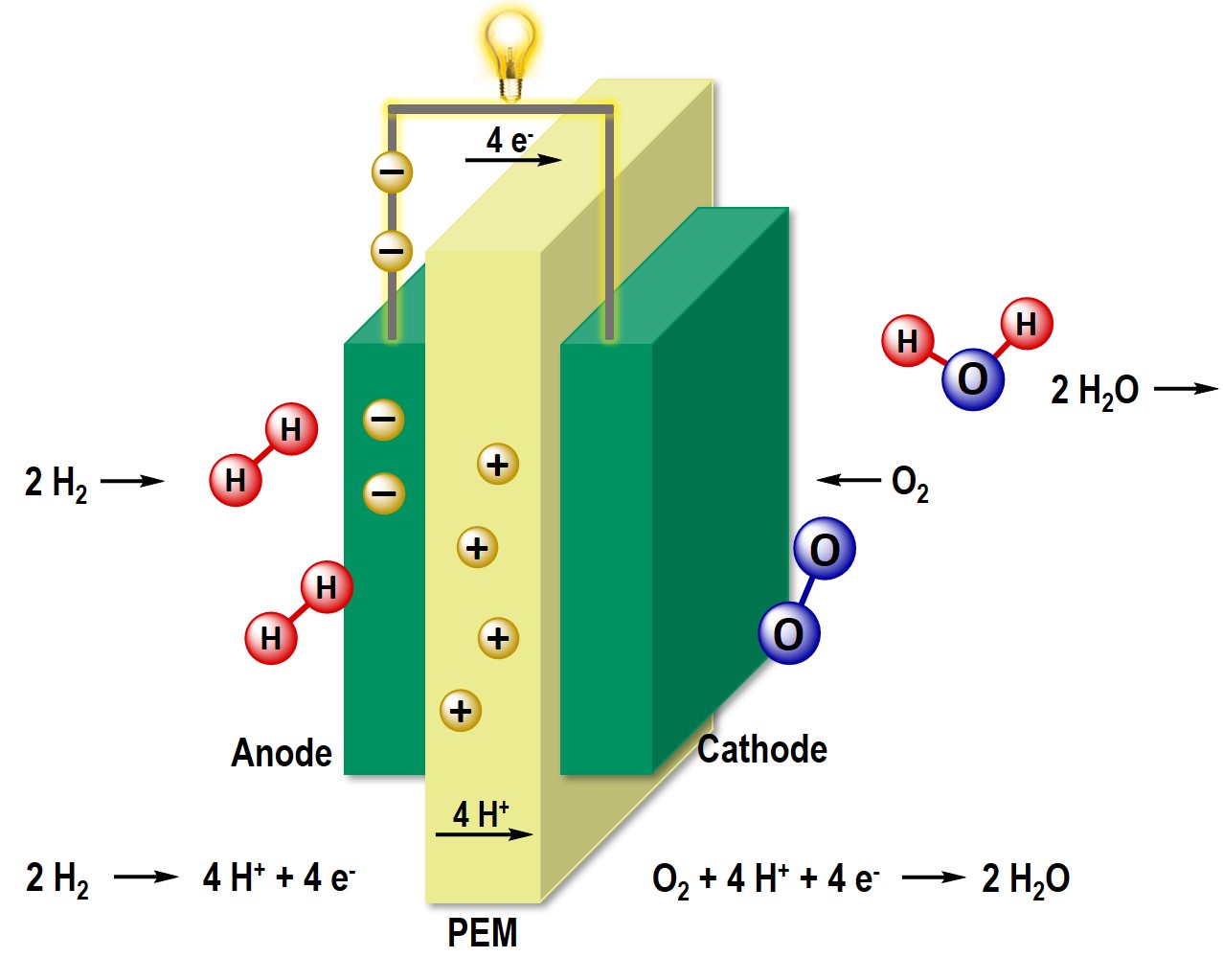
The use of fuel cells as energy converters with high thermodynamic efficiency offers a climate-friendly and low-emission alternative to fossil fuels. Thus, the fuel cell is at the centre of the current hydrogen strategy of the Federal Government. Our research focuses on the synthesis of precious metal-based and non-precious metal-based nanostructured catalyst materials for electrochemical oxygen reduction. We are particularly interested in the so-called HT-PEM fuel cell, which, among other things, has the advantage of a higher operating temperature. We investigate the poisoning of the active Pt centers in operation with X-ray absorption spectroscopy and try to influence this positively by using organic additives.
Direct reductive conversion of CO2
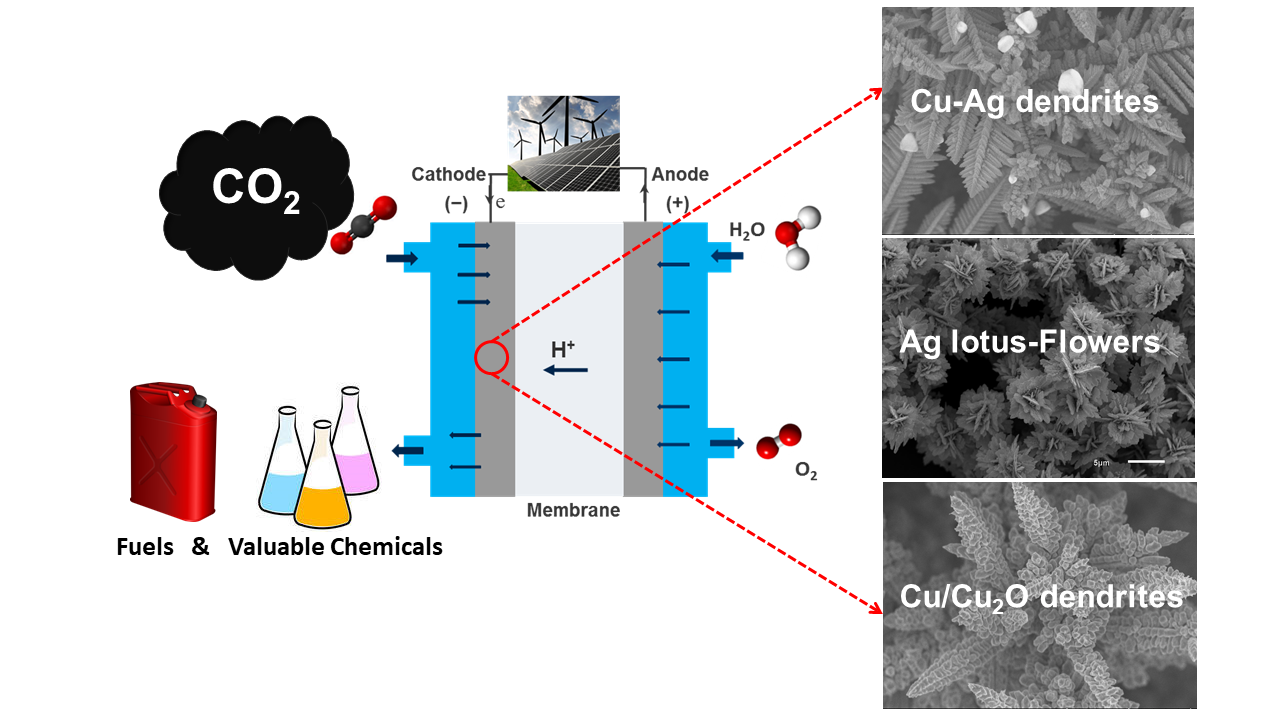
For the chemical-material storage of renewable electricity, water electrolysis is to be directly coupled with the conversion of carbon dioxide (CO2) into higher-value products (e.g. CO, C2H4, CH3OH). Copper in particular is suitable as a catalyst for CO2 electrochemical reduction. In a current project we use Cu and CuAg films produced by negative hydrogen bubble tempering as model systems to analyze the influence of composition and pore size on the resulting products.
Operando X-ray Absorption Spectroscopy (XAS)
Our research focuses on the application of operando X-ray Absorption Spectroscopy (XAS) to study the structural and electronic dynamics of catalysts under realistic working conditions. Using synchrotron facilities, we combine XAS with electrochemical techniques to investigate crucial parameters such as oxidation states, coordination environments, and adsorbed species. This approach provides valuable insights into catalyst activation, conditioning and degradation mechanisms, and helps with the design of more durable and efficient materials for energy conversion systems, including fuel cells, water electrolysis, and CO₂ reduction reaction.
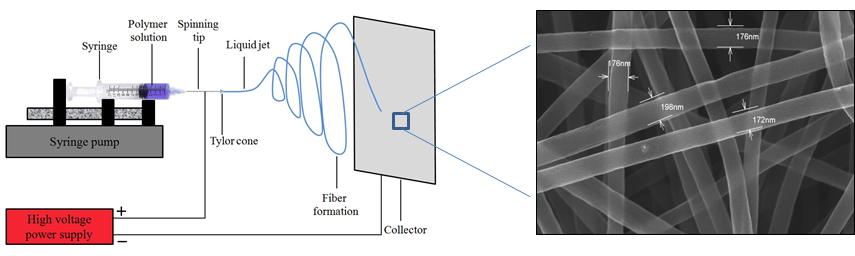
Electrospinning process
The electrospinning process is a method that can be varied in many ways to produce fibre structures with very high specific surfaces. Electrospun PAN felts can be carbonized and used in fuel cells, redox flow cells or in other electrochemical energy applications where highly porous materials are used. In our group we use electrospinning to produce fine-fibrous carbon networks and hollow fiber structures and investigate the combination of polymer fibers with metals.